Melody Watson and Dr. Marc Esser (veröffentlicht in Medical Writing 2016; 25(4):31-33)
Writing, publishing, and disseminating a medical review
Have you been commissioned to write a review? Reviews are useful for drawing attention to issues and benefits related to a product. One thing they are not, however, is something to be feared.
What is a review?
A review is a critical analysis that brings together published literature or data within a specific subject. There are several different types of reviews. In narrative reviews, authors summarise the literature, compare studies, discuss data and develop new hypotheses. Status quo reviews contain only the most recent research. Systematic reviews address a clear question using systematic methods to select and evaluate relevant studies and may include statistical analyses, including meta-analyses.1 In general, as a medical writer you should be able to write most types of literature-based reviews. However, for systemic reviews that include statistical analyses or meta-analyses, you will need to have a statistical background or employ the help of a statistician.
Why are reviews important?
A review draws attention to a company’s drug or medical device and strengthens a company’s scientific profile. For instance, publishing a review when a product is through primary clinical trials, but has not yet been approved, creates a pre-marketing buzz around the product. Additionally, following approval, a review helps establish the product within a treatment paradigm. Reviews are also useful when a product is older and interest in it has consequently declined or if there are specific features or concerns surrounding a product. For example, if a drug is associated with particular adverse events, you could write a review that provides information on how to best manage these adverse events. Another approach could be to report on how the drug or the medical device works in actual clinical practice, using real-world data. This is often interesting for physicians, as clinical trials are highly controlled and are therefore not representative of the actual environment in which a drug is being administered.2
Planning the review
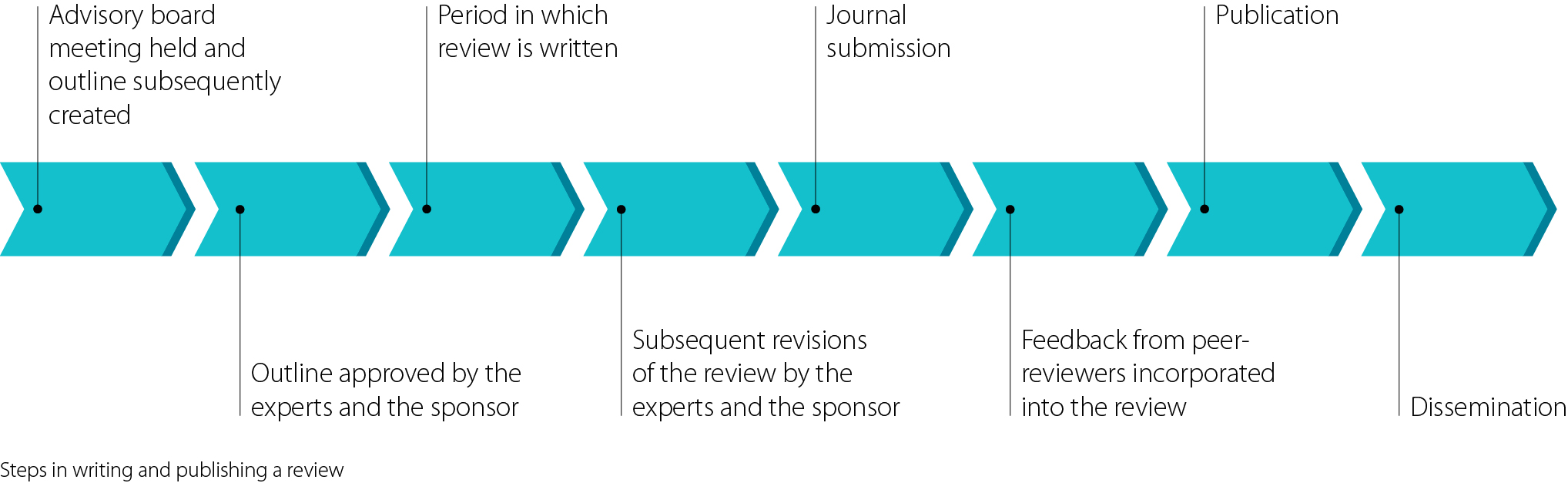
Generally, the first step towards writing a review is holding an advisory board (adboard) meeting comprising experts, sponsor representatives (for example, from a pharmaceutical company), and a medical writer or two. It is crucial to match the right experts to the topic being covered and to include at least one key opinion leader. A key opinion leader is a thought leader in his or her field and is usually someone who has published pivotal research in top-tier journals or authored important textbooks. Key opinion leaders are included because they provide valuable insights and lend legitimacy to the efficacy and safety of a company’s product.
During the advisory board meeting, different topics will be discussed with different input from various stakeholders. As a medical writer, your main role is to note the key points for the yet-to-be-written review, distilling the discussion into clear goals for the review, yet capturing the nuances of what is being said. You may also have to lead the group of experts through the agenda to reach a consensus, make sure the meeting remains on time, and define each author’s role and responsibilities within the project. Following the advisory board meeting, you will write up a brief describing the aims of the review and send it to the experts and sponsor for approval. Sometimes it can be hard to balance what the sponsor wants with what the experts are willing to say about a product, but in the end an agreement will usually be reached. Getting the brief right is crucial to writing a good review, or at least one that does not have to undergo too many corrections by the experts and the sponsor. Often, the medical writer is also expected to write an executive summary following the meeting. This will be sent to the sponsor.
During the advisory board meeting, there should also be a discussion about which journal the review should be published in. Fitting a publication to a target journal is no easy task. Both the pharmaceutical company and the experts will want the review to be published in a journal with a high impact factor. However, this must be balanced by how relevant the review actually is and how realistic publication in a high-impact journal would be. In order to avoid endless revisions for various journals, have a realistic approach from the beginning.4 In addition to traditional journals, open-access journals are another option for publication. Although they may sometimes have lower impact factors, this is compensated for by having a wider audience for distribution and dissemination of your article.
Writing the review
Before beginning to write, go to the target journal’s website and look for the webpage containing the instructions for authors. On this page you will be given instructions on how to prepare your document, such as the word count, line spacing, which form of English to use (if submitting to an English language-based journal), and how many tables and/or figures are permitted. It is much better to do this at the beginning of the review rather than at the end. A checklist covering the requirements for submission may also be included in the instructions for authors.
The first draft of a review usually takes between one and two weeks to write. At the beginning, take notes while reading through the literature, recording insights on how you might organise the review and collecting interesting pieces of information and thoughts on what you might write. As a result, you will have a rough draft of the review early on. This can help with motivation.4
You should try to use as much primary research as possible. Include high-quality studies, pivotal trials, and (not too many) other reviews. Inclusion of unpublished data should be the exception. Think about what is relevant for your topic. If the review is about a first-in-class drug, then mode of action matters. Think about your target audience. If the review is about a new formulation of a well-established drug, pharmacokinetic and pharmacodynamic studies might be interesting for physicians. Use studies that are current, but do not forget to use older studies that still contain valuable information. Additionally, do not just summarise the literature, instead care fully discuss it, pointing out methodological strengths and weaknesses. After reading your review, a reader should have an idea of the important achievements in the field, the major topics that are under debate, and which research questions are still outstanding.5
Include tables and figures where appropriate. Figures are particularly useful if you are trying to explain a complicated mechanism of action of a drug. For this, you will need to employ the services of a graphic designer experienced in illustrating medical topics. If you include tables, make sure they supplement the text and do not simply repeat it. This specification is often included in a journal’s instructions for authors, but it is a good habit to adopt in general.
Write the abstract last. After completing the main text, you will be familiar with the content and tone of the manuscript making this task much easier than if you had attempted to write it at the beginning of the process. Selecting accurate keywords is essential for correct indexing and for getting your review to the right audience. Note terms used repeatedly in the text and terms that most appropriately describe your review, then check that they can be found in the appropriate indexing standard. In medicine, this is called Medical Subject Headings (MeSH), a National Library of Medicine-controlled vocabulary thesaurus.
After finishing the abstract and selecting the key words, send the manuscript to all of the stakeholders. They will then review it to make sure it fits with their objectives. Depending on the size of the expert group, it can take several weeks, or even months, for all of the experts to read the review, make suggestions, and send it back. Additionally, it can sometimes take several revisions for the experts and the sponsor to shape a manuscript that is acceptable to all parties. Keep in mind, however, that the experts have the last say as to what the manuscript contains.
The final steps
Sometimes a journal will request a cover letter to accompany your manuscript. Specifications for the cover letter are usually in the instructions for authors. They normally require you to state that your manuscript has not been published or is not under review elsewhere. You should also state why you feel your manuscript is important, interesting, and a good fit for the journal. Keep the cover letter clear and concise – journal editors may read dozens of cover letters per day and skim over cover letters longer than a few paragraphs. Now your manuscript (plus cover letter) is ready to be submitted to the target journal.
The submission process
Following journal submission, an editor will screen the manuscript and decide whether or not your manuscript is an appropriate match for their journal. You will hear back from the editor about this first decision relatively quickly. If the editor decides to consider your review it will be sent to at least two peer reviewers. The peer review process can be completely open, single-blind (the names of the reviewers are not revealed to the authors), or double-blind (neither the names of the reviewers nor the authors are revealed to one another). As with the initial stages, in which the experts reviewed the manuscript, peer review can last for months depending on how many reviewers have been selected and what their work schedule is like.
The editor will return your manuscript accompanied by comments from the peer reviewers; seldom are manuscripts accepted for publication without any requested changes. Peer review is another point in the publication process in which your manuscript may be either rejected or accepted.6 Make use of the feedback! In general, comments from peer reviewers can really help to improve your manuscript, especially as they are seeing it with fresh eyes. Peer review comments can be very direct and sometimes it can be hard to not react to negative comments, especially if you consider them to be unfair. However, try to respond politely. If you feel as though the peer reviewer has completely misunderstood your review or overlooked a crucial feature, then you can discuss this with the editor and request another review.7
After making the changes requested by the peer reviewers, send your revised manuscript back to the authors for their final approval. Following approval, send it back to the journal along with the responses to each peer review comment. If your manuscript is accepted without any further changes needing to be made, congratulations! If your manuscript is rejected, either in the beginning of the submission process or following peer review, then it is time to look for another journal, maybe one with a lower impact factor or a scope that better fits your review.8 As with the first journal, you will have to format your document to fit the requirements listed in the new journal’s instructions for authors. With respect to reformatting references, this is quite easy nowadays with reference management software such as Endnote.
Distribution
Following publication, it is imperative to ensure that the review reaches as many members of the target audience as possible. It is safe to assume that not every member of the target group will have a subscription to the journal the review has been published in. However, there are other ways to disseminate the review, such as including it on the sponsor’s web page, using offprints in trade fair booths and having the sales force give offprints to physicians.
Conclusion
Writing a review is a relatively straight-forward process that can be initiated whether or not there are new data for a product. Furthermore, reviews have more credibility, more leverage, and draw more attention to a product than marketing materials such as brochures. Remember, the key to writing a good review is its foundation – a clear brief containing the various opinions of the stakeholders. Reviews are nothing to fear – as long as you approach the process in an organised and patient manner, with attention to detail (skills that most medical writers possess), not much can go wrong.
References
1. Mayer P. Guidelines for writing a review article. 2009. Available from: http://www.plantscience.ethz.ch/education/Masters/courses/Scientific_Writing
2. Mahajan R. Real-world data: Additional source for making clinical decisions. Int J App Basic Med Res. 2015;5(2):82.
3. Moynihan R. Key opinion leaders: independent experts of drug representatives in disguise? Br Med J. 2008;336(7658):1402-3.
4. John M. The target journal: Choosing the right place to submit your paper. HSR Proc in Intensive Care Cardiovasc Anesth. 2009;1(3):60-2.
5. Pautasso M. Ten simple rules for writing a literature review. PLoS Comput Biol. 2013;9(7):e1003149.
6. Voight ML. Publishing your work in a journal: Understanding the peer review process. Int J Sports Phys Ther. 2012;7(5):452-60.
7. Wager E, Godlee F, Jefferson T. How to Survive Peer Review. BMJ Books. Pub. 2011. Available from: http://www.bmj.com/sites/default/files/ attachements/resources/2011/07/ wager.pdf
8. Ali J. Manuscript rejection: Causes and remedies. J Young Pharm. 2010;2(1):3-6.